COVID-19 - December 28, 2020
By Amber McCracken
America has never backed down from a fight. Our history is full of heroic, victorious battles. Today, however, our country finds itself combating a very different foe. The enemy this time is invisible and stealthy. It makes its way into our homes, hurting those we love. The old adage of “look your enemy in the eye” does not apply, yet everywhere we look, we see its effects on all of us.
We have been waging the war on the novel coronavirus for a year now. Has the secret weapon to end it arrived in the form of a vaccine? Many believe it might be, and with the vaccine we might be able to claim victory in the months to come.
According to Johns Hopkins University’s Coronavirus Resource Center, as of December 17th, we’ve seen over 74 million cases of COVID-19 worldwide, and we have lost 1.6 million people globally. The United States has had close to 17 million cases and over 307,000 deaths. It is no wonder that the entire research community has raced to find the silver bullet of vaccines to stop this enemy in its tracks.
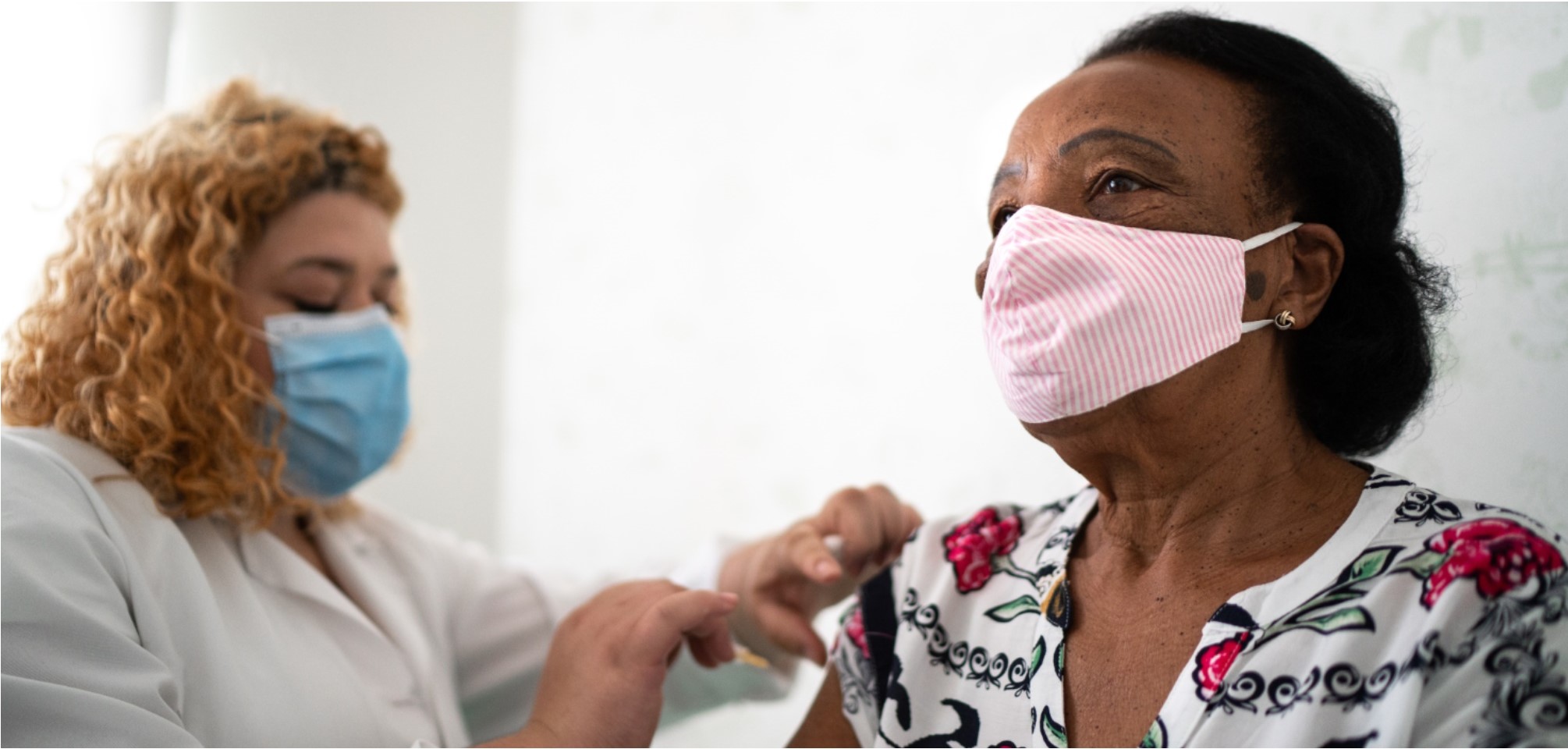
On Sunday, December 13, the first shipments of the Pfizer vaccine left the company’s manufacturing warehouse in Michigan and have now been distributed all across our country. The images of frontline health workers receiving their first shot have flooded our screens, rousing celebration and inspiring hope that our long battle may be coming to an end.
While most are applauding the arrival of the Pfizer vaccine (with others, including one from Moderna, fast on its heels), some are concerned at the “warp speed” at which it arrived. If you ask the scientific soldiers in the field, they have been working non-stop on a vaccine since China released the SARS CoV-2 full genome on January 10. This genome was the first significant piece of the research puzzle. Even before the U.S. had its first reported COVID-19 patient on January 21st, scientists and researchers from here and abroad had started the arduous task of mapping out a possible vaccine.
Research and development of vaccines and other medical advances take time. If you consider vaccines for polio, smallpox and measles, we have waited years, even decades, for a vaccine to come to market. Prior to the COVID-19 vaccine, the fastest vaccine ever developed was for mumps—it took four years to develop. While the COVID-19 vaccine represents something unprecedented and perhaps a new model for creating vaccines in the future, it is also rooted firmly in the past, one of significant scientific progress.
In a recent appearance on ABC’s Good Morning America, Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID), remarked “The speed was the reflection of extraordinary advances in the science of vaccine platform technology to be able to do things technically in months that some time ago – five, 10 years ago – may have taken several years.”
According to the Centers for Disease Control and Prevention (CDC), the general stages of vaccine development are:
The CDC states that “Clinical development is a three-phase process. During Phase I, small groups of people receive the trial vaccine. In Phase II, the clinical study is expanded and vaccine is given to people who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended. In Phase III, the vaccine is given to thousands of people and tested for efficacy and safety.”
All companies currently working on a COVID-19 vaccine are following all of these steps. What’s different this time is the gravity of the situation. Due to the pressing, worldwide needs, many companies are working on the manufacturing of the vaccine at the same time as the clinical development phase in order to be ready for massive distribution.
In the same interview, Dr. Fauci remarked on how effective the manufacturing model has been for distribution. “The investment of hundreds of millions, if not billions, of dollars in getting the vaccine ready for distribution also contributed to the speed in getting the medication out.”
The amount of funding in support of a vaccine is unparalleled as well. Operation Warp Speed, the Trump Administration’s initiative to accelerate the development of a vaccine, awarded Moderna $955 million to advance its clinical trials, and another $1.5 billion to manufacture and deliver 100 million vaccine doses. Pfizer’s contract is different: The company received $1.95 billion to manufacture and distribute 100 million doses, but it did not accept funding for research or development.
After a long summer and fall of uncertainty, Pfizer and their partner BioNTech announced that their vaccine had a 90% efficacy on November 9. This news came after their Phase III trials, which included 44,000 participants, representing a diverse cohort. Similarly, on November 16th, Moderna announced a 94.5% efficacy for their vaccine after their Phase III trials (click here to learn more about efficacy). Pfizer’s vaccine received FDA approval for emergency use on December 11, and millions of doses were released the following week, most administered to frontline healthcare providers across the country. Moderna’s vaccine received approval on December 18.
According to this New York Times article, “Pfizer’s chief executive has said that it could have 30 to 40 million doses of the vaccine before the end of the year, enough for 15 to 20 million people to get an initial shot and a booster three weeks later.”
The CDC notes that the most common side effects of the vaccines include pain and swelling on the arm where you get the shot, as well as fever, chills, tiredness and headaches. The efficacy was the same across the board for all demographic populations, racial and ethnic backgrounds and ages. It is also important to understand that the vaccine does not contain a live virus, a misnomer being falsely circulated. In other words, you cannot get COVID-19 from receiving the vaccine.
Now the pressing question is: Will the American public take the vaccine? The vaccine has been highly politicized, leading many to worry about its safety. According to Gallup, in November, only 58% of Americans would agree to be vaccinated against COVID-19. A Harvard University School of Public Health survey found that only 14% of African Americans and 34% of Latinx would take the vaccine, which is especially troubling considering that these populations of color have been disproportionately affected by the pandemic.
However, in a more recent ABC News poll, more than eight in 10 Americans say they would receive the vaccine, with 40% saying they would take it as soon as it’s available to them and 44% saying they would wait a bit before getting it.
The 40% willing to take the vaccine clearly falls way short of the estimated 70 – 80% of Americans needed to take the vaccine in order to achieve herd immunity. According to the Mayo Clinic, “Herd immunity occurs when a large portion of a community (the herd) becomes immune to a disease, making the spread of disease from person to person unlikely. As a result, the whole community becomes protected — not just those who are immune.”
Each state across the country will determine its own distribution plan for the vaccine. Most states will likely adhere to the CDC’s recommendations, which suggest that a Phase I distribution roll out prioritizes groups such as healthcare workers, residents of long-term care facilities, essential workers and any adult living with a chronic condition.
To that end, Goodwin Living has been notified by the Virginia Department of Health (VDH) that our health care center residents and the staff serving health care residents will be among the first to receive the COVID-19 vaccine. Our first vaccine clinics will be administered through CVS COVID-19 vaccination teams at Goodwin House Alexandria on Dec. 29 and Goodwin House Bailey’s Crossroads on Dec. 30. We are in regular contact with VDH and CVS in our effort to secure the vaccine for all Goodwin Living residents, Goodwin Living At Home members and staff.
While Goodwin Living residents and staff will be encouraged to get the vaccine, any who have medical or religious concerns about being vaccinated may meet with infection control nurses and the Goodwin Living executive director of their campus to discuss their concerns. Many staff and residents and ready and eager to get vaccinated.
“The science says, ‘Go for it!’ Dr. Fauci says, ‘Go for it!’ You bet, I will get the vaccine!” shared Zainab Thomas, a nurse practitioner who works at Goodwin House Bailey’s Crossroads. “This vaccine is backed overwhelmingly by science. Let’s heed the call and CRUSH this devastating disease once and for all. I will take it without a doubt!”
——–
Amber McCracken is the executive director of Current Communications, a boutique consultancy that helps organizations with their marketing and public relations activities. Amber has worked with GHI since 2014, providing her expert advice to support Goodwin Living At Home. She contributes regularly to The Good Life, both as a writer and editor. Amber lives in North Carolina with her husband and two children.
